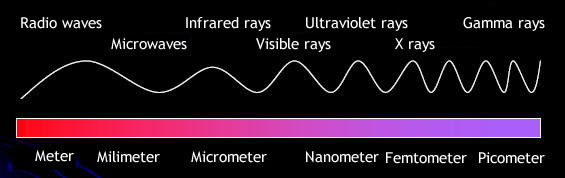
Infrared Rays
Infrared (IR) lights are electromagnetic radiation with thier wavelength longer than those of visible lights,
measured from the nominal edge of visible red light at 0.74 micrometers, and extending conventionally to 300 micrometres.
(In the visible light region, lights' color change from red to violet as their wavelength become shorter, and finally become invisible again. Then come the Ultraviolet (UV) lights whose wavelengths are longer than X-rays,
ranging 10 nm to 400 nm. Rays ranging from 0.01 to 10 nanometers are classified into X-rays.
Electromagnetic rays whose wavelength are shorter than X-rays are called Gamma rays.)
FT-IR spectroscopy
FT-IR Spectroscopy is the abbreviation of "Fourier Transform Infrared Spectroscopy". This is the analytical technique developed in 1970s to qualify and quantify compounds utilizing infrared absorption of molcules.
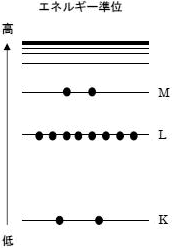
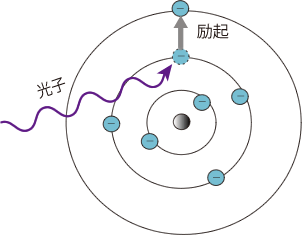
Absorption occurs when the energy of the beam of light (photons) are transferred to the molecule. The molecule gets ”excited ”and moves to a “higher” energy state. The energy transfer takes place in the form of electron ring shifts, molecular bond vibrations, rotations, and translations. IR is mostly concerned with vibrations and stretching.
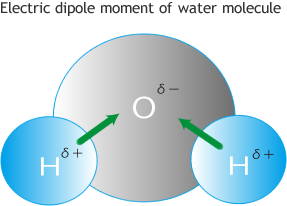
A molecule is infra-red active if it possesses modes of vibration that cause a change in dipole moment.
Prerequisite for molecules to have dipole moment is either
- Molucules have permanent dipoles: These occur when two atoms in a molecule have substantially different electronegativity: One atom attracts electrons more than another, becoming more
negative, while the other atom becomes more positive. See dipole-attractions.
(Quoted from Wikipedia, http://en.wikipedia.org/wiki/Dipole)
- Dipole moment is induced in a molucule which has no permanental dipole but through molucular vibration (asymmetrical streching or bending vibration)
Regarding prerequisite 1
No difference in electronegativity
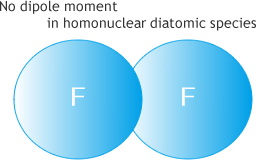
Difference of electronegativity of constituent atoms induces dipole moment
As shown in the above image, monatomic and homonuclear diatomic species (N2, O2, F2, H2, Cl2, etc) are infra-red inactive.Then how do you think about molecules which are symetrical but not homonuclear?
CO2, composed of heteronuclear atomes, has no parmanent dipole moment since electronegativity vectors are cancelled each other.
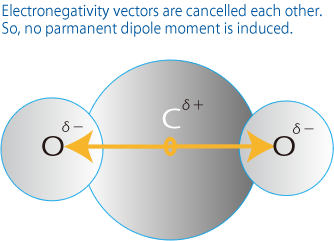
But it does have dipole moment based because of molucular vibration (asymmetrical streching or bending vibration). [Prerequisite 2],
Asymmetrical streching
Bending vibration
How these compounds vibrate or stretch are unique depending on sizes and kinds of constituent atoms. IR peaks of compounds are unique either. FTIR spectroscopy, based on uniqueness of IR peaks, make following studies available
- Study of structure, distribution, and reactions of molucules, ions, and crystals.
- Reserch of condition of molecular excitation or reaction of intermediate.
- Qualitative and Quantitative Analysis
- Monitoring of reaction process
Reference: Iundustrial Publishing and Consulting, Inc. "Jitsuyou Bunkouhou Series─Sekigai Bunkouhou" Yukihiro Ozaki
Nowadays, many FT-IR analyzers are available from various suppiler, and especially solid and liquid phase FTIR spectrometer are popular.
MIDAC CORPORATION is the company who has been engaged in gas-phase FT-IR spectrometer.
Its FT-IR gas analyzers are epoch-making instrument for process monitoring of CVD, Etcher, and scrubbesr in semiconductor industry and established its rock-solid position as a de facto standard machine in the field. Hongo Inc. is the exclusive distributor of the company in Japan.
Basic FTIR theory(1)
What is FT-IR?
Basic FTIR theory(2)
Basic FTIR theory(3)




